Warning Letter Highlights Importance of CGMP Requirements By Ryan Maus
Failure to Develop a Foreign Supplier Verification Program - Top FDA Citation for Fiscal Year 2024
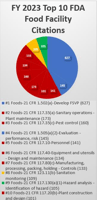
According to FDA’s Data Dashboard, the most frequently cited violation by FDA investigators in fiscal year (FY) 2024 (ending September 30, 2024) was failure to develop a Foreign Supplier Verification Program (FSVP). This is the top violation cited since FY 2018 when FDA began enforcing FSVP rule compliance. According to FDA data, warning letters issued for FSVP violations in FY 2024 decreased from the previous year. However, they still made up 23% of the 146 warning letters issued to food manufacturers. In the past month alone, seven warning letters were published when food importers failed to correct FSVP violations cited during FDA investigations.

Six of those did not develop an FSVP. Normally, FDA investigators will determine if foreign food is imported during routine investigations. Manufacturers that import food and lack FSVP documentation will be cited for not following the FSVP rule if no exemptions apply. Six of the seven warning letters published this past month cited inaction on developing, maintaining, and following an FSVP as required by section 805 of the FD&C Act and 21 CFR 1.502(a) for any of the foods
they import. Generally a written response to a warning letter is required within 15 working days of notification. If the matter is not adequately addressed, the FDA may take further action including an FDA product hold, detention, and Import Alerts (e.g. Import Alert # 99-41). Removal from an Import Alert is a lengthy process. The importer is required to submit information to the FDA that adequately demonstrates resolution of the conditions that gave rise to the FSVP violation and provide assurance that FSVP
requirements will be met for future imports.
Final guidance is available from FDA to help importers comply with the FSVP rule. U.S. importers who have responsibility for fulfilling FSMA FSVP requirements for foreign suppliers that manufacture, process, pack,
or hold food intended for human or animal consumption in the U.S. must use a qualified individual to develop and perform FSVP activities. These activities include determining known or reasonably foreseeable hazards associated with each type of food, evaluating risks posed by the supplier’s performance, approving suppliers, conducting appropriate supplier verification activities, and verifying corrective actions that provide assurance that the food’s hazards have been significantly minimized or
prevented. Detailed records documenting these activities are also required.
The Food Safety Preventive Controls Alliance (FSPCA) developed a course that provides participants with the knowledge to implement the requirements of the “Foreign Supplier Verification Programs (FSVP) for Importers of Food for Humans and Animals” regulation of the FDA. The FSVP curriculum was designed by regulatory, academia, and industry professionals and with funding from FDA. Deibel Laboratories has several FSPCA trained FSVP lead instructors and offers this course. Please see our website for course registration information.